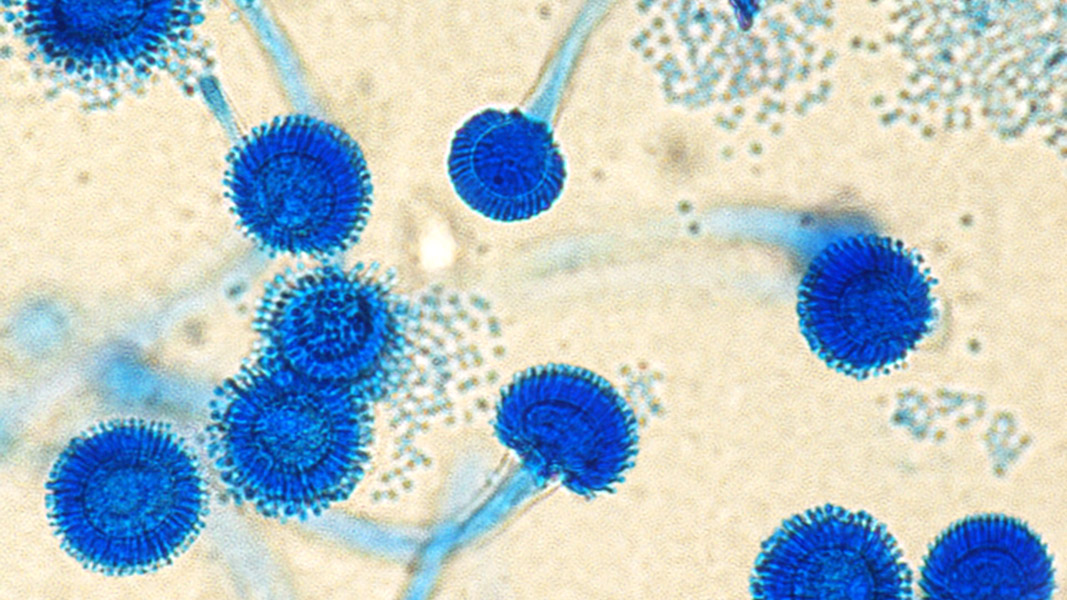
Top: Aspergillus Fumigatus. Image courtesy Ufficio Comunicazione, Azienda Ospedaliera SS. Antonio e Biagio e Cesare Arrigo, Alessandria and Biblioteca Biomedica Centro di Documentazione, CC BY 4.0, via Wikimedia Commons (cropped and manipulated by BioCycle).
Craig Coker and Patricia D. Millner
A recent news headline — “Critical Public Health Threat: Deadly Fungus Discovered in Commercial Soil, Compost, and Flower Bulbs” — brought to the forefront a decades-old reality for the composting industry. Journalistic hyperbole aside, it was important to dig into this story to see if this is Aspergillus fumigatus déjà vu.
A. fumigatus became a serious issue for composting facilities in the 1970s and 1980s and was associated with development of composting solutions for wastewater treatment sludges (more on this below). A. fumigatus is a ubiquitous saprophyte (meaning it grows on decaying organic matter) and a human-pathogenic fungus that is life-threatening to immunocompromised individuals. It causes over 250,000 invasive infections each year and is found in soils, plant debris, mulches, and compost (Celia-Sanchez et al., 2024), as well as in agricultural work environments (Kofoed et al., 2024).
Human Health Effects
The primary route of infection by Aspergillus is inhalation of airborne spores (conidia) and their deposition in the lung’s bronchioles or alveolar spaces. The average size of A. fumigatus conidia (2 to 3 μm) is ideal for infiltrating deep into lung alveoli. Figure 1 shows how the fungus grows on laboratory agar plates (Fig. 1, a-d), and how the spore-bearing filaments (known as conidiophores, Fig. 1 e-g) and conidia (the smallest blue ‘dots’ shown) appear microscopically.
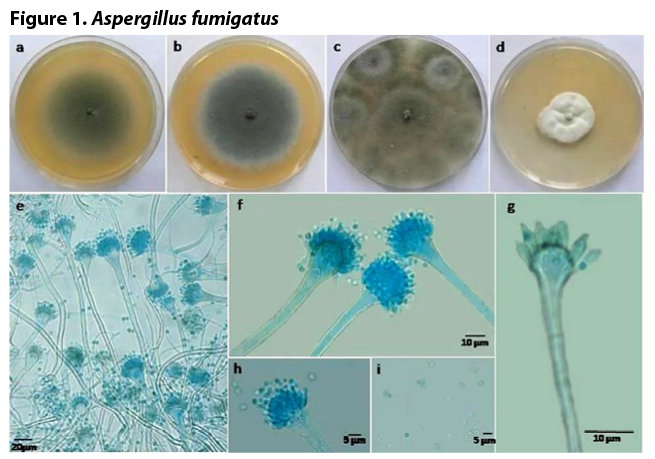
Figure: Aspergillus fumigatus. a, b. Seven days colonies of autumn (A.PZ1) and spring (A.BZ1) isolates on Malt Extract Agar plates; c, d. Seven days colonies of autumn (A.PZ1) and spring (A.BZ1) isolates on Czapek Yeast Agar plates; e, f. conidiophores; g. vesicle and phialides; h, i. conidiophores with conidia. Image Source: Mycologia Iranica – Khamesay, S.J., et.al., “Identification of the fungi absorbing heavy metals isolated from waste deposits of zinc factories.” Used with permission of Prof. Dr. Amir Hossein Hamidian
Spores of A. fumigatus, along with other airborne fungi and bacteria, are inhaled without causing problems in healthy individuals. However, for people who are immunocompromised, or who have chronic respiratory diseases or conditions, such as cystic fibrosis, tuberculosis, sarcoidosis, and patients with Coronavirus disease 2019 (COVID-19), the spores can germinate and infect the lungs. Among the bioaerosols (i.e., airborne biological materials) emitted from composting facilities (A. fumigatus is a fungal type of bioaerosol), the principal potential types of health effects in humans identified include, but are not limited to the following (Pearson, 2015):
- Allergic asthma, rhinitis, hypersensitivity pneumonitis/extrinsic allergic alveolitis, allergic bronchopulmonary aspergillosis, eye and skin irritations
- Toxic non-allergic asthma, mucous membrane irritations, chronic bronchitis, chronic airway obstruction such as chronic obstructive pulmonary disease (COPD), organic dust toxic syndrome (ODTS), toxic pneumonitis
- Infectious aspergillosis, zygomycosis; immunocompromised individuals are more susceptible at lower concentrations of the relevant pathogens
Azole-Resistant A. Fumigatus
The eye-catching headline was based on a recent report documenting the presence of triazole-resistant A. fumigatus in retail products (Wang et al., 2024). While azole-based compounds, which are 14-alpha demethylase inhibitor (DMI) fungicides, are among the most effective treatments available for A. fumigatus infections, they are also highly valuable for control of plant pathogens on multiple types of crops. Evidence has now accumulated that after four decades of wide-spread agricultural use of azole-based compounds as crop protection fungicides, azole resistance has emerged in clinically and environmentally isolated A. fumigatus as well as in closely related Aspergillus strains (Burks et al., 2021; Jørgensen and Heick, 2021; Badali et al., 2021; Dladla et al., 2022; Morrissey et al., 2024). Indeed, the World Health Organization (WHO) has recently issued the first fungal priority pathogens list (FPPL) to raise awareness of the risk of fungal infections and the increasingly rapid spread of antifungal resistance.
While azole drugs are among the most effective treatments for A. fumigatus and several other clinically relevant Aspergillus and fungal infections, their extensive use in agriculture as fungicides has led to a critical adverse effect on azole-based treatment efficacy in healthcare settings. Azole-resistant A. fumigatus has been increasing in Europe and Asia for two decades. The clinical resistance has been related to the extensive agricultural use of azole fungicides (Celia-Sanchez et al., 2024).
In the Wang study, researchers collected grapes, apples, almonds, pecans, peanuts, compost, soil, and flower bulbs from eight retail grocery stores and nine garden centers in the Athens, Georgia area. Flower bulbs and compost were identified as dense sources of azole-resistant A. fumigatus and were reservoirs and potential drivers of long-distance dispersal of antifungal-resistant isolates. They also identified that retail raw peanuts, almonds, and pecans were potential reservoirs of fungicide-resistant A. fumigatus, concluding that these food items and landscape products could pose a risk to people who are immunocompromised or have decreased lung function. Several major recent review publications have highlighted the worldwide increase in azole-resistant A. fumigatus isolated from patients.
Aspergillus In Composting
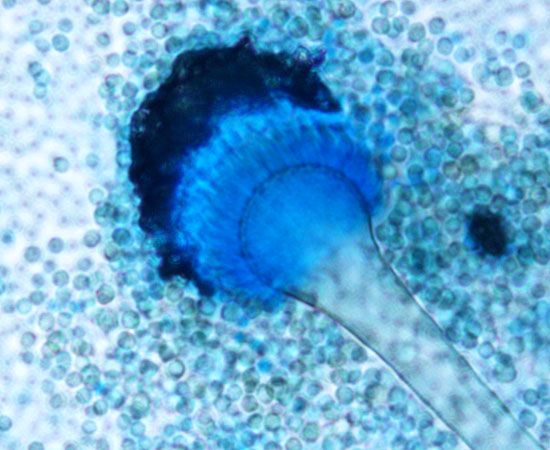
Aspergillus fumigatus. Image courtesy Ufficio Comunicazione, Azienda Ospedaliera SS. Antonio e Biagio e Cesare Arrigo, Alessandria and Biblioteca Biomedica Centro di Documentazione, CC BY-SA 4.0, via Wikimedia Commons (cropped and manipulated by BioCycle).
Some of the most definitive research on A. fumigatus in composting was conducted at the U.S. Department of Agriculture’s Agricultural Research Service in Beltsville, MD. In one study (Millner et al., 1977), A. fumigatus was regularly isolated as a dominant fungus during forced aeration composting and after 30 days in an unaerated stationary curing pile; in both cases, the fungus was found in pile zones with temperatures less than 60oC (140oF). Compost stored outdoors in stationary unaerated piles from one to four months after screening out woodchips contained easily detectable amounts of A. fumigatus in the exterior pile zones (0- to 10-in. depths). Semiquantitative studies of the airborne spores at the composting site revealed that A. fumigatus constituted 75% of the total viable mycoflora captured. At locations 320 m (1,049 ft.) to 8 km (≈ 5 miles) from the compost site, the fungus constituted only 2% of the total viable mycoflora in the air.
In a subsequent study (Millner et al., 1980), the researchers measured A. fumigatus concentrations in the air at the Beltsville Agricultural Research Center composting facility. Aerosols of A. fumigatus downwind from stationary compost piles were insignificant in comparison with those downwind from agitated piles. These aerosols were generated by a front-end loader (FEL) moving and dropping compost. The ranges of aerial concentrations at the downwind sites were as follows (in number of particles per cubic meter of air): total, 17 to 55,563; actinomycetes, 7 to 15,301; fungi 24 to 55,521; A. fumigatus, 7 to 55,021. The survey at the compost site indicated that the intensity of aerosolization was mainly associated with mechanical movements of the compost piles. After the FEL stopped moving compost, the A. fumigatus aerosol at 3 and 30 meters (9 and 90 feet) downwind of the piles was approximately 33 to 1,800 times less concentrated than that measured during movements and not significantly above background levels.
The results of the survey indicated that a variety of thermophilic actinomycetes and fungi were also aerosolized. Some of these actinomycetes are known to incite hypersensitivity reactions in the human respiratory system when inhaled in large numbers on a recurring exposure basis. The measured concentrations of thermophilic actinomycetes (maximum, 15,000 actinomycete particles per m3), however, were not nearly as high as the maximum of 15 x 109 actinomycete particles per m3 found by previous investigators studying such aerosols in barns during the shaking of moldy hay.
Another study (Browne et al., 2001) involved the collection of health symptom data and environmental monitoring data near a 40-acre grass and leaf composting facility near Islip, NY. Analyses were based on symptom diary data from 63 individuals from the study area and 82 individuals from a reference area. The study area was a neighborhood northeast of the composting facility and was selected based on prevailing wind direction (making it the most likely to be exposed to A. fumigatus transported by air from the composting facility). The reference area was five miles from the facility and not subjected to the prevailing wind. Airborne A. fumigatus was not associated with reported increases in respiratory or irritative symptoms. Symptom incidence was associated with ragweed, ozone, temperature, and time since start of the study, although a tendency to report fewer symptoms as the study progressed may have confounded this result. The results of this study suggest that if elevated concentrations of A. fumigatus spores due to operations at the composting facility are causing increases in allergy and asthma symptom prevalence, the increase was too small to detect given the study limitations.
Management Options
The four main methods of dealing with A. fumigatus at composting facilities are 1) Explanation and transparency of working conditions during employee candidate interviews and before hiring — including safety/health practices and procedures at the work site; 2) Efficient and insightful site design; 3) Conscientious site operation; and 4) Proactive response to new research findings.
Employee Hiring
Prospective employees deserve to be informed formally about the nature of the worksite materials, exposures, and potential known health impacts. Employers and employees would document that the work conditions have been explained and that safety and health trainings are provided by the employer. Employees are required to satisfactorily complete and observe the procedures appropriate for their assigned positions and responsibilities. All employees need to be informed and understand the conditions and potential safety and health conditions and risks associated with the positions they would or do occupy.
It is illegal to ask a job applicant about their medical history (EEOC, 2024). An employer may not ask a job applicant, for example, if he or she has a disability (or about the nature of an obvious disability). An employer also may not ask a job applicant to answer medical questions or take a medical exam before making a job offer. An employer may ask a job applicant whether they can perform the job and how they would perform the job. The law allows an employer to condition a job offer on the applicant answering certain medical questions or successfully passing a medical exam, but only if all new employees in the same job are required to answer the same questions or take the exam.
Composting Facility And Equipment Design
Good site design includes adequate vegetated buffers between composting operations and any nearby sensitive receptors (e.g., public gathering areas) and an understanding of how meteorological and topographic features affect on-site air flows and transfer of airborne particulates generated on-site to adjacent off-site areas. Vegetated buffer strips are known to trap and filter particulates and bioaerosols are often associated with dust particles. Containerized and enclosed composting and screening processes provide options for mitigation of fugitive dust release. Air-conditioned cabs for operators and provision of appropriate filtration systems in such equipment, and evaluation of the efficacy of exposure reduction resulting from use of various protective equipment, are needed.
Conscientious Site Operation
Good operational procedures and approaches to site operation include: 1) Turning compost based on temperature, not schedule; 2) Restricting material movement to periods when the potential for off-site airborne transfer (i.e., low windspeed) and receptor populations are minimal (time of day, avoid weekends and holidays); 3) Mitigating dusty conditions with water sprays; and 4) Providing appropriate Personal Protective Equipment (PPE) to all employees working in areas where compost is being agitated (moved, turned, screened) and others who may request PPE.
Proactive Responses To New Research
The reported presence of azole-resistant A. fumigatus in compost products (and thus its likely presence at composting facilities), as well as documented soils and agricultural settings, provide managers an opportunity to establish a proactive response by: a) Incorporating this new information into their facility safety and health training documents and operating practices; b) Considering what additional information and actions may be needed to provide quality products in the marketplace while informing both employees and the general public about safe product handling practices; and c) Supporting/advocating for research and development of antifungal agents dedicated for use in agricultural settings as distinguished from those for use in healthcare settings.
Craig Coker is co-owner of Star City Compost LLC in Roanoke VA, a senior editor at BioCycle Connect, and CEO of Coker Composting and Consulting. He can be reached at ccoker@cokercompost.com. Pat Millner is a Research Microbiologist with the U.S. Department of Agriculture, Agricultural Research Service, Beltsville, Maryland. She can be reached at pat.millner@usda.gov.