Sally Brown
BioCycle August 2016
I knew that I was ready to be done with graduate school the fifth time a professor in five different classes taught me about the nitrogen cycle. In agronomy and soils, you learn about nitrogen (N) so much because it is so important and so hard to pin down. Nitrogen is typically the plant nutrient in greatest demand and traditionally it has been the one in the shortest supply. Plants need it to grow and so do people. Proteins all have N in their structures — and you know how important protein is to our diets. No one can survive on potato chips alone.
Nitrogen is also tough because it changes. It can be a solid or a gas. It can be locked up and immobile. It can also flow through soil like water through a sieve. And when it is a gas, it can be multiple kinds of gases, from innocuous to one of the most harmful greenhouse gases. It can also be the basis of regulations for land application of organics. In other words, it is high time for a nitrogen primer. I promise no tests at the end.
Atmospheric
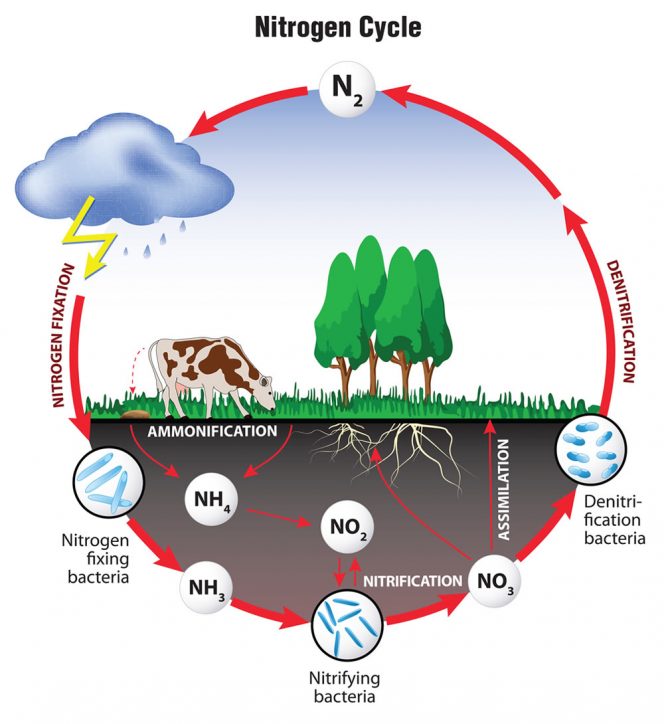
Let’s start with the atmosphere. About 75 percent of the air is nitrogen gas. By nitrogen gas I mean N2. While plants and people are good at getting oxygen from the air (even though it is a measly 17 percent of the atmosphere), neither plants nor people can absorb the nitrogen gas. To access that N, it has to be changed from a gas to a solid form, which requires a lot of energy.
The key to this reaction is changing the valence state, i.e., the number of electrons circling around the N nucleus. No atom wants those electrons and so they have to be forced on. One of the ways this transformation occurs in nature is with lightning. The electrical energy in the lightning bolt is powerful enough to turn the gas into a solid by shoving electrons onto the molecule. But we can’t always count on lightning and it isn’t really a good idea to try to get close to those bolts.
The most important natural N conversions are done by bacteria. These bacteria — rhizobia, frankia and some algae — are able to convert the gas to a solid or mineral form via a process called mineralization. They take nitrogen gas and turn it into ammonium (NH4+). In other words, the neutral N gas gets three electrons dumped on it. This requires a lot of energy. To get that energy, these bacteria make friends with plants and take some of the sugars and carbohydrates from photosynthesis. In exchange, they give them some of the nitrogen that they “fix.”
The host plants are typically called legumes and come in a range of shapes and sizes. Beans are legumes, which is one of the reasons they are good for you. They have a lot of protein. Alfalfa is another, which is why it is good for cows and horses. In fact, farmers plant legumes and other crops together as a way to get enough nitrogen for those other crops. But not everyone wants to put lentils in with their corn.
In come the Germans in World War I. The chemists Fritz Haber and Carl Bosch figured out how to put those electrons on the gas using energy. In fact, depending on the source of energy used, it takes about 4 units of CO2 to get one unit of ammonia. The reason that Haber and Bosch did this was to make explosives, namely TNT or trinitrotoluene. In fact, certain kinds of nitrogen fertilizers also make great explosives, which is why it became impossible to buy ammonium nitrate — the most popular nitrogen fertilizer — after the Oklahoma City bombing.
Figuring out how to make ammonia fertilizer was the basis for the green revolution in agriculture. Once the N2 gas gets into a solid ammonia form, it becomes easy for plants and animals to access. It also becomes very easy for those same animals to transform it — and this is the part where you make the transition from a straight line (N2 gas to NH4+ solid or mineral) — to those nitrogen cycle circles. Those circles all revolve around the N losing some of those electrons and eventually turning back into a gas.
Nitrification
The next thing that happens to the N once it is ammonium is that microbes change it into nitrate (NO3-). This step is called nitrification. That is a huge change in the electron balance, going from having three extra to being 5 short. What that means is that the bacteria that do this work get a lot of energy for their efforts. This is a two- stage reaction, going first from ammonium to nitrite and then from nitrite (NO2-) to nitrate. And it is a fast reaction. Nitrate is the form most plants like. It is also the form that moves through soil very quickly — causing the dead zone in the Gulf of Mexico and other eutrophication problems.
This critical reaction is important because it yields the form of N that is the most important for plant nutrition and also the most potentially hazardous for the environment. Almost all of the N-based regulations are promulgated to prevent the release of excess NO3- or nitrate into the environment. In addition, sometimes this reaction doesn’t go all the way to nitrate and you can end up losing nitrogen back to the atmosphere as a gas. Not the good neutral N2 gas we breathe in every day, but rather the evil 296 times the potency of CO2 nitrous oxide (N2O) gas. That typically happens when there isn’t a lot of oxygen around. It is not thought to happen all that often, but it can happen.
What if you have nitrate and it hasn’t leached or turned into nitrous oxide? It can get turned back into the good neutral N2 gas by microbes in a process called denitrification. This is generally a good thing. It typically happens in environments where there is very little oxygen. Wetlands are a good example. In fact, denitrification — or turning the potentially environmentally harmful nitrate back into the neutral, most-of-the-atmosphere-consists-of-it gas, is one of the primary benefits of wetlands. This reaction dumps electrons back onto the nitrate. However, because this process involves multiple electrons, sometimes it doesn’t always work all the way. As a result, this is the most important pathway for formation of nitrous oxide. It also explains why high clay soils that can easily be saturated and low in oxygen are a source of N2O.
Role Of Organics
Where do organics come into all of this? Organic N is the other critical form of nitrogen. The nitrogen in plants, people, manures, food waste and biosolids is primarily in organic forms. That means it is coordinated with carbon, phosphorus, oxygen and other elements as proteins. While not as hard to access as N2 gas in the atmosphere, organic N is not readily plant available or leachable or transformed into N2O. In order for the organic N to be transformed to mineral N (you guessed it, this process is called mineralization), the organic matter that contains it must be eaten. The critters doing the eating are typically eating for the same reasons we do — energy and nutrients.
How much of that N gets released from the organic form will depend on how much carbon it is tied up with. If there is too much C in relation to N, none of the organic N is released. In fact, if you have a soil with too much N fertilizer you can add carbon to it and immobilize the excess N. Only if there is extra N in relation to C will you get an N surplus and the transformation to ammonium.
On a very basic level, our urine is how we get rid of much of the excess N we consume. That is one of the reasons that urine makes a great fertilizer. The N in the pee is in the form of urea, which is highly plant available. In contrast, the N in the poop is organic N, and it requires a much longer time to transform. The change from organic N to mineral N is a relatively slow reaction — typically taking several months to years. This is the critical difference between fertilizing with synthetic versus organic (meaning carbon based) materials. The synthetic nutrients are typically ammonium (di-ammonium phosphate aka DAP or mono ammonium phosphate aka MAP or plain ammonium or urea). These are very quickly transformed into nitrate and can be lost from the system in minutes to days.
Nitrogen in organic forms will transform only as the carbon itself is also mineralized and will be released to the soil only when there is more than enough N to satiate the consumer. That happens in days to years. What this means is that adding 100 lbs of N to a soil as ammonium and adding the equivalent amount in the form of compost, manures or biosolids, will yield very different amounts of plant and environmentally relevant N. We can’t say exactly how much available N you get from organics but we can do a decent job of estimating. And the loss potential with these estimates is much lower than with synthetic fertilizers.
In other words, if used at close to predicted agronomic rates, organic sources of N have very little potential to cause environmental harm. That is the end of the lecture. And while there is no test, hopefully you can see that feeding your soil a balanced slow release diet is the best way to close the loop and complete the nitrogen cycle.
Sally Brown is a Research Associate Professor at the University of Washington in Seattle and a member of BioCycle’s Editorial Board.