New testing methods may be available by mid-2014, including molecularly imprinted polymers that simplify extraction and analysis procedures, and a “dipstick” to test for the presence, although not necessarily the concentration, of certain persistent herbicides. Part III
Craig Coker
BioCycle January 2014
Previous articles in this series (June 2013 and August 2013) have discussed the 2012 recurrence of persistent herbicide contamination of compost in Vermont and steps composters can take to manage the risks of dealing with contaminated feedstocks and compost. Persistent herbicides (PHs) are a class of herbicides that include pyridine carboxylic acid herbicides (also called plant-growth regulators or auxin-mimics) and they can be bioactive at levels as low as 1 part per billion (ppb), or roughly one-half teaspoon in an Olympic-sized swimming pool. These herbicides are not degraded fast enough in the composting process to reach nonbioactive levels in the normal time frame for composting. Problems with contaminated composts adversely affecting horticultural crops have been reported by several facilities after first surfacing in 1999.
Testing, or characterizing, feedstocks for composting is a standard operating procedure at many facilities. Feedstocks are normally tested for parameters important to composting recipe development, such as carbon and nitrogen concentrations and moisture content. Testing is also often done for parameters that can affect final product quality, such as heavy metals and soluble salts. These tests are normally conducted with standardized chemical analysis procedures. Numerous private laboratories around the country can do the analyses, as well as many of the State Agriculture laboratories.
However, testing for PHs is very difficult. One reason is that feedstocks and composts are considered complex matrices for laboratory analysis (a simple matrix would be a sugar solution in water). The term matrix refers to the components of a sample other than the chemical being sought (the analyte); the matrix can influence both how the sample is analyzed and the results obtained. Analyzing for PHs requires that the PH be extracted from the sample using solvents. Extraction solvents can be water, water/methanol mixtures, or one of several proprietary formulations. “If composts are produced with proper attention to good process management, the herbicide residue can be extracted with water with a revised extraction procedure we are developing,” explains Mike Hastings, a Principal Research Scientist with Dow AgroSciences, which manufactures PHs including picloram, clopyralid and aminopyralid. “The difficulty is that when you extract compost with water, you get compost tea, and both the color and other compounds in the extracted solution cause complications with the analysis.” In the case of feedstocks like grass clippings and vegetative material, the herbicide can bind inside the plant tissues, requiring more complex extraction techniques. This new analytical procedure being developed by Dow AgroSciences is in final development and will be published for use by State agricultural and other laboratories by the middle of 2014.
Hastings notes that because these herbicide compounds have small molecular weights and are highly polar (meaning they display a high affinity for the electrostatic attraction between two oppositely charged ions), they can be difficult to separate out following extraction. In the case of herbicide analysis, separating the herbicide from an extracted sample means passing the sample through solid-phase extraction cartridges to eliminate the complicating impurities. Once separated, the sample is then analyzed using liquid chromatography and mass spectrometry procedures. The equipment needed to analyze for these herbicides at detection levels corresponding to the levels of contaminant concern to composters is very expensive and not all commercial or state agriculture laboratories have that equipment. “A high-end laboratory with this type of equipment and well-trained chemists could analyze for these compounds, but it will likely cost $150-$200 per sample,” he adds.
Another consideration is that State agricultural laboratories are tasked with a different mission. “Over 90 percent of the workload in state pesticide labs is to support the enforcement programs work related to drift issues or other pesticide misuse investigation cases,” explains Cary Giguere, the Agrichemical Management Section Chief for the Vermont Department of Agriculture. “Most State agriculture labs really were not designed to do work-for-hire for composters or others. We were able to help investigate and analyze the trace-back sources of the herbicides that affected Green Mountain Compost, but that was part of our enforcement investigation.” Chittenden Solid Waste District’s Green Mountain Compost facility in Williston, Vermont had problems with PH contamination in 2012. (See Part I, “Unraveling The Maze of Persistent Herbicides In Compost,” June 2013.)
The advantage of spending the money to have a sample of a feedstock tested is that the composter can manage the PH residue through recipe algebra (see “Composters Defend Against Persistent Herbicides, Part II,” August 2013). The obvious disadvantage is deciding on whether to disclose a level of contamination in the final product going to market, particularly if it is below a No Observed Adverse Effect Level (NOAEL). The NOAEL is the highest tested concentration of a substance (like a drug or a chemical) at which no adverse effect is observed where higher concentrations would show an adverse effect. For example, assume the concentration of the herbicide was low enough so as not to damage the plants in a bioassay test conducted at the composting facility. Should the numerical value of that concentration be reported? One of the challenges in releasing information about the constituents in composts is that many people do not understand the meaning of the chemical data in laboratory reports (this has been demonstrated many times by potential customers of biosolids composts who have reviewed and questioned heavy metal concentrations in the final product quality analysis).
Additional Test Methods
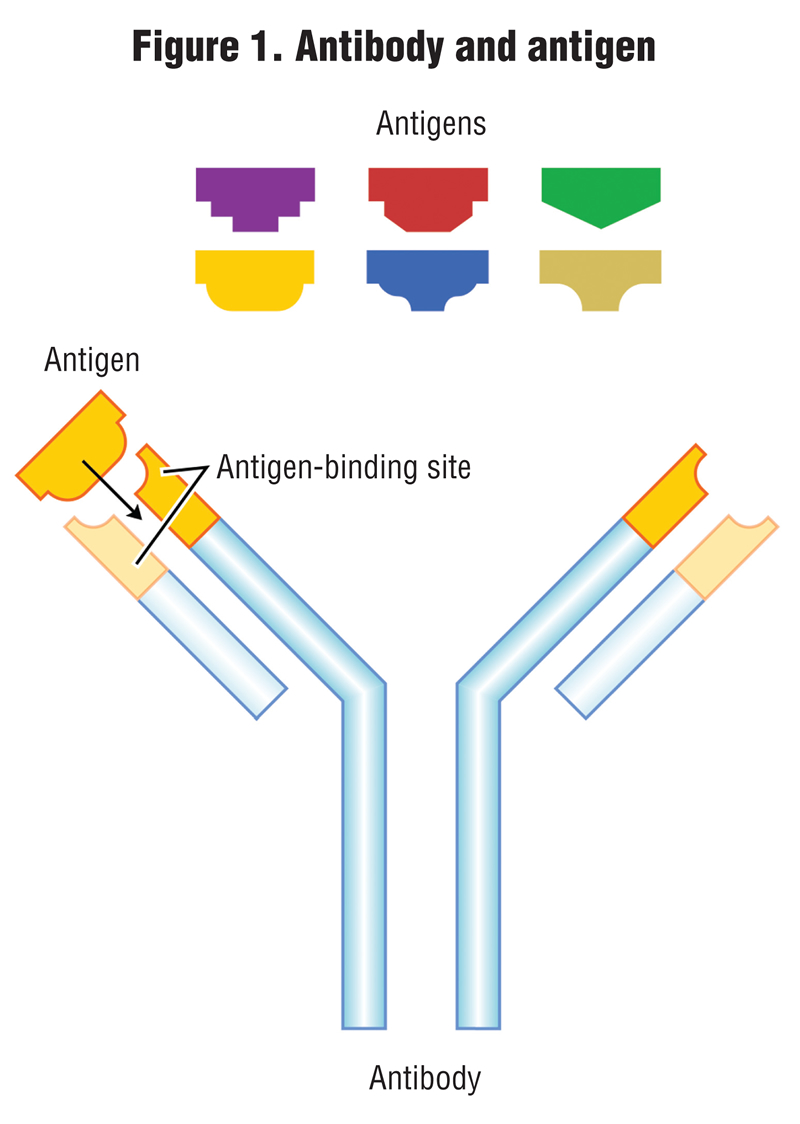
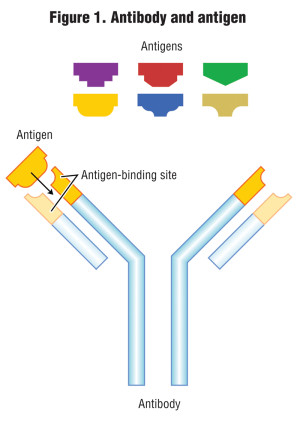
The other methods of testing for PHs are biological; one is called immunoassay, and the other is a bioassay test. An immunoassay method measures the concentration of a chemical (known as an antigen) in a solution through the use of antibodies, where the antibody recognizes and binds to that specific chemical (antigen) and to link the antibody to some sort of detectable label, like a color change or fluorescence under black light. The process is analogous to how the human body produces antibodies to counter invading bacteria or viruses. Antibodies and their respective antigens fit together like a key and lock (Figure 1).
There has been some discussion in both industry and regulatory groups about the potential to develop rapid-response testing using an immunoassay procedure known as enzyme-linked immunosorbent assay (ELISA). This would be analogous to the Solvita™ stability tests practiced by many composters. An ELISA test works on the principle of detection of an antigen in a liquid sample by a detection antibody linked to an enzyme (a catalyst) and its substrate (the chemical upon which the enzyme acts); the reaction between the antibody, the enzyme and the substrate produces a visible signal, such as a color change. ELISA testing is used in medical evaluations (for HIV, West Nile virus, tuberculosis antibodies etc.) and in detecting potential food allergens (milk, peanuts, walnuts, almonds, eggs). ELISA can be done in a laboratory or in the field. Field testing is done with a procedure known as Lateral Flow Immunochromatographic Assays, which is the same technology used in home pregnancy test kits, malaria tests, avian flu tests and other applications. To date, no commercial ELISA test kits are on the market for pyridine carboxylic acid herbicides but some research into developing such a kit has been funded by the U.S. Department of Agriculture.
The other biological testing method is bioassay. Bioassay testing is a procedure where the concentration or potency of a substance is measured by its effect on living cells or tissues. Many of the laboratories that composters use for product quality analysis can do germination testing for product maturity, which is a form of bioassay. Bioassay testing is being used by Green Mountain Compost in Vermont. Composters should realize that doing in-house bioassays requires 90 to 100 growing pots (to measure effects of different ratios of composts and soils and to have duplicate and triplicate pots to repeat and verify results) and two to three hours per week to properly analyze for impacts. Bioassays, when set up and run properly, are the best method for evaluating the potential impacts from herbicides. But there are a number of potential interferences from salinity, boron content, pH and maturity that can create false positives and false negatives.

Figure 2. Woods End® Laboratories’ Plant-Injury Risk Management (PIRM) bioassay testing for herbicide impacts
Final Assessment
So, where do we go from here? The reality for the composting industry is that these PHs are not going away, and chemical/biological testing for them is going to remain expensive. Therefore, composters should develop management protocols for dealing with them. Options include refusing to accept potentially problematic feedstocks, developing in-house bioassay tools (or using a commercial lab to do the bioassay), and seeking expansion of the prohibition on PH usage in a state, which can be done by working with the herbicide producer and the appropriate Association of American Pesticide Control Organization (AAPCO) official in each state (www.aapco.org/).
Other initiatives to consider include development of a “chain-of-custody” program for problematic feedstocks, similar to the ones in place for handling hazardous chemicals. The composting facility, which is the last stop in the chain, would have details on where the feedstocks were sourced. “One challenge to developing a chain-of-custody program is deciding who would run the program,” notes Giguere. “Maybe that is something a nonprofit organization could do, similar to how the organic agriculture certification program is run?” Another idea being discussed is a chain-of-custody program offered as product stewardship with the producers of PHs, but that will be complicated by the fact that some of these herbicides have gone off-patent protection and there are now numerous producers.
Work is underway to develop faster analytical procedures, to understand the extent of PH degradation during active composting and to continue the ongoing dialogues between industry officials, state agriculture departments and composters. This type of collaboration will be important to help composters manage their feedstocks and their facilities in the future.
Craig Coker is a Contributing Editor to BioCycle and a Principal in the firm Coker Composting & Consulting (www.cokercompost.com), near Roanoke, VA. He can be reached at cscoker@verizon.net.