Sally Brown, Andrew Carpenter and Lauren Souther
Top: SCAQMD 25.3 flux chamber and sample train (helium carrier gas, impinger, summa canisters). Photo courtesy of ECS
Over the past decade, more and more regulatory agencies are requiring that composters measure both volatile organic compounds (VOCs) and ammonia emissions. This started in California, in regions where Air Quality Management Districts (AQMDs) were not meeting air quality standards for reducing smog. Quantifying air emissions is becoming an increasingly common component in siting and operating permits for composting facilities in the U.S. and Canada. In other words, these requirements are not going away in the near future. In fact, they may be coming to an air quality district near your operation.
While VOCs include odorous compounds, they can’t be measured by a simple sniff test. Compost operators have been monitoring emissions of odorous compounds as a way to keep neighbors happy and their gates open for a long time. Measuring emissions for regulatory compliance is a different deal. A majority of regulations are concerned with all non-methane, non-ethane organic compounds (NMNEOC) and ammonia, whether they smell like rotten eggs or perfume. The given basis for these regulations is that a subset of VOCs and ammonia can react with nitrogen oxide (NOx) to form ground-level ozone and smog. Some VOCs emitted by composting are on the EPA’s list of Hazardous Air Pollutants (HAPs) and are therefore of regulatory concern (Table 1), and others (namely methane) can impact greenhouse gas emissions. It is important to realize that approximately 80% of the VOCs coming off of compost piles are light alcohols with minimal odor, human health impact or smog forming potential (Green 2010).
 Where Do VOCs Come From?
Where Do VOCs Come From?
VOC emissions from composting will vary based on feedstocks, temperature, level of aeration, pH, point in the composting process and just about any other variable you can think of. That is the case for VOCs in general. For specific compounds or specific VOCs, certain conditions are required for emissions to become significant. Methane (CH4) is an example of a VOC that is only produced in cases of low available oxygen. You are likely to detect CH4 early in the composting process or in an uncovered pile in areas with high rainfall.
The same conditions are required for the broad class of odor compounds that contain reduced sulfur. Examples of those include dimethyldisulfide and hydrogen sulfide. Nitrogen-based emissions require different conditions. Ammonia (NH3), a concern for both odors and health, is typically emitted later in the composting process, when organic nitrogen has been mineralized by microbes. Ammonia becomes a gas at higher pH levels, or when a pile is basic (alkaline) rather than acidic. Direct sunlight will also increase NH3 emissions.
Nitrous oxide, a concern for carbon accounting, also comes later on in the composting process. It also requires the nitrogen to have transformed, in this case one step past NH3, to nitrate (NO3-). Nitrous oxide can be formed when that nitrate is converted to nitrogen gas, typically under saturated conditions. While in general the highest VOC emissions occur early in the composting process, even at the tip floor, that is not the case for all VOCs.
There have been a number of scientific studies on emissions of VOCs from composting operations. Most of these have measured emissions from research-sized composting units where environmental conditions are controlled. One study looked at the impact of process control variables on emissions of different VOCs (Delgado-Rodríguez et al., 2011). Most, although not all, emissions were found in the early stages of the composting process. Researchers found that more aeration meant higher emissions, and that emissions reduced over time and were generally lower in piles with higher C:N ratios. Other studies have shown that sufficient aeration is key to reducing odors, a critical class of VOCs (Gage, 2003). A study of emissions from different parts of the pile found much higher emissions from readings collected from the top of the pile rather than on the sides (Büyüksönmez, 2011).
 These studies have also shown that there are differences in emission rates based on the specific VOC being measured. There is insufficient data on VOC emissions to allow for more than the most basic conclusion: Much more at the beginning but still some during the process. One thing that research has been consistent about is the efficacy of biofilters to reduce VOC emissions (Iranpour et al., 2005).
These studies have also shown that there are differences in emission rates based on the specific VOC being measured. There is insufficient data on VOC emissions to allow for more than the most basic conclusion: Much more at the beginning but still some during the process. One thing that research has been consistent about is the efficacy of biofilters to reduce VOC emissions (Iranpour et al., 2005).
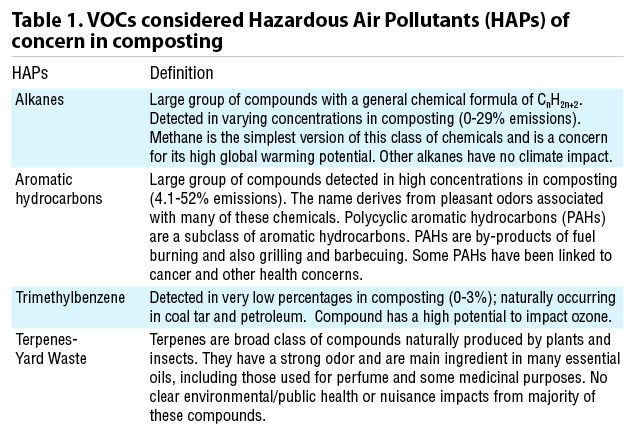
Figure 1 is a profile of VOCs over time from a green waste composting operation. These samples were collected using wind tunnels and flux chambers with gas collected in Summa canisters and charcoal tubes and analyzed with gas chromatography-mass spectrometry (GC MS). About 90% of the measured VOCs were non-ozone-forming (Kumar et al., 2011).
How To Measure VOCs
The first issue with sampling ammonia and VOCs is where to collect the sample. Here options fall into three categories:
- Direct emissions: Consist of air samples taken from the surface of a compost pile or biofilter with sampling equipment in physical contact with the feedstocks. These are point-in-time measurements from a very small area and provide a snapshot rather than a full picture of compost emissions.
- Indirect emissions: Consist of samples collected somewhere within a composting operation but not in direct contact with the pile. While indirect samples collect a fraction of the total air in a facility during a limited time, they will reflect some of the variation across a site.
- Fenceline: Refers to samples collected from the property boundary. This monitoring has most typically been done to assess odors from a facility. For VOCs, fenceline sampling has all of the errors associated with direct and indirect sampling, with more additional air and wind thrown in.
The second issue with measuring VOCs and ammonia is how to actually collect the air sample. The methodologies for sample collection and analysis that have become the de facto standard methods for many composting facilities in the western U.S. are the South Coast (CA) Air Quality Management District (SCAQMD) Methods 25.3 and 207.1, for NMNEOCs and ammonia, respectively. These methods are a modified EPA flux chamber method outlined in SCAQMD’s Rule 1133.3 Emission Reductions from Greenwaste Composting Operations. Figure 2 provides an illustration of Method 25.3.
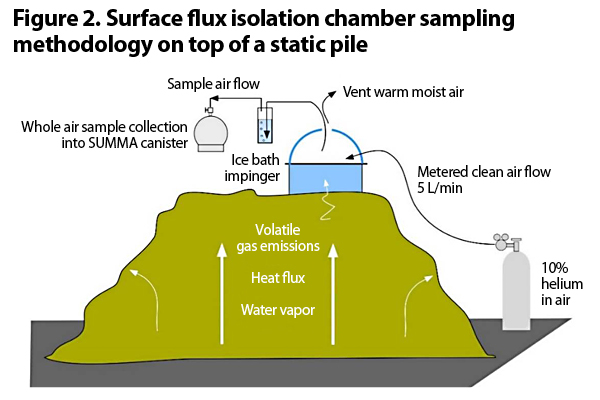 This method calls for flux chambers, which are small typically open bottom cylinders that you place on a compost pile. Emissions from the test surface are isolated within this volume, mixed with an inert carrier gas, and sampled. The surface area covered by a standard flux chamber is 0.13 m2 or 1.4 ft2 (Kumar et al., 2011). Flux chambers can be used on both aerated and unaerated surfaces.
This method calls for flux chambers, which are small typically open bottom cylinders that you place on a compost pile. Emissions from the test surface are isolated within this volume, mixed with an inert carrier gas, and sampled. The surface area covered by a standard flux chamber is 0.13 m2 or 1.4 ft2 (Kumar et al., 2011). Flux chambers can be used on both aerated and unaerated surfaces.
Wind tunnels are similar to flux chambers but larger. They cover feet of the pile rather than inches. They can also have air forced through them when sampling an unaerated surface. Wind tunnels have gained some acceptance as a substitute for standard flux chambers.
The third issue is how to extract the samples from these chambers or tunnels. As the air is pushed through the chamber or tunnel, all methods require some type of trapping mechanism to collect the VOCs for later measurement. One protocol (SCAQMD 23.5) requires that you collect samples over a 60-minute period. As air enters the chambers, it is pushed through condensate traps to capture low concentration VOCs while the remainder is pushed into the canister. This means that there are two samples to analyze for VOCs. Ammonia is often collected and analyzed separately.
Method TO-15 requires you to collect air for 6 minutes. The air is pumped into a stainless steel canister. A known volume is forced through a solid multi-sorbent concentrator that also reduces water content. The concentrator is then heated in a lab to desorb the gases, which are analyzed using a gas chromatograph.
The final issue is how to measure the sample once it’s collected. Almost all measurement techniques require transport of the sample to a specialized lab for analysis. Regulations typically require analysis of total NMNEOCs rather than quantification of specific gases and their concentrations. A gas chromatograph flame ionization detector (GC FID) takes the resin used to collect the sample and heats it so that all of the sorbed compounds volatilize. This gives a value for total NMNEOCs.
If you are looking for quantification of specific compounds, you need a gas chromatograph mass spectrophotometer (GC MS). This works on a similar principle as the GC FID but is able to compare emissions spectra from individual gases to known standards. Sample analysis with a GC MS is more expensive than with a GC FID. This type of analysis is not required by current regulations.
Some advanced methods exist that allow for real time measurements. For sampling within a facility you can use a hand held PID/FID (PID is a photo ionization detector) to determine gross emissions, electronic noses that can be calibrated to estimate a range of compounds in place, or sorbent tubes that are compound specific. These are relatively new methods that are not recognized by current regulations. There are also options for measuring the air around a facility. An example is a micrometeorological mass balance (MMB). Towers are set up around an emissions source and emissions as well as wind speed and direction are measured to come up with a general flux measure. To date, this has been used for research and is not recognized for regulations.
To sum up Part I, composting emits a wide range of VOCs. A portion of these can impact air quality. Emissions will vary based on feedstocks, type of composting system and where and how you collect samples. Regulations require that these be measured and controlled. Next up in Part II is testing to meet compost air emissions regulations.
Sally Brown, BioCycle’s Senior Adviser and long-time Connections columnist, is a Research Professor at the College of the Environment at the University of Washington. Andrew Carpenter is a certified soil scientist, certified crop advisor and certified nutrient management planning specialist. He founded Northern Tilth, an environmental consulting firm focusing on organic waste management and building soil health, in 2003 and has been recycling organic matter-based by-products since 1992. Lauren Souther is a research scientist and nutrient management planning specialist at Northern Tilth. She received a BS in Wildlife Biology from Unity College in 2017.
Authors’ Note: This article series is based on a report developed for the Composting Council Research and Education Foundation (CCREF) and funded with donations from the composting industry.













